miraDry
miraDry was cleared by the U.S. FDA in January 2011 to treat axillary hyperhidrosis or excessive underarm sweating and is now available in North America, Asia Pacific, and Europe. (Looking for miraDry near you? Use this miraDry locator).
The device was also FDA-cleared in 2015 to permanently reduce unwanted underarm hair of all colors; making it the first FDA-cleared device available to halt both unwanted underarm sweat and hair growth. (Note that for hair reduction, the device is sometimes called miraSmooth instead of miraDry.) For our readers, we’ll focus on miraDry’s sweat-stopping capabilities. If you want more information on permanent hair-zapping, visit www.mirasmooth.com. Just a note on this though, a benefit of miraDry/miraSmooth for permenant hair removal is that it works no matter what color your skin or hair is, which is a benefit it has over laser hair removal.
A study published in the Journal of Cosmetic and Laser Therapy in 2013 showed that miraDry was effective in treating both axillary (underarm) hyperhidrosis and axillary osmidrosis (foul-smelling sweat). As part of the study, 11 patients in Asia were treated with miraDry and then evaluated 7 months later for improvement of symptoms. At the follow-up, 83.3% of the underarms treated were determined to have experienced a 2 point improvement in the Hyperhidrosis Disease Severity Scale (HDSS). Among those underarms that had exhibited foul odor (osmidrosis), 93.8% showed good to excellent results.
In of April 2012, clinical data from the University of British Columbia showed that miraDry was successful in reducing underarm sweat in over 90% of patients. The average sweat reduction was 82%. Patients rated their satisfaction with the treatment at 90%.
Recently, Carolyn Jacob, MD wrote the following in an article published in the March 2013 edition of peer-reviewedSeminars in Cutaneous Medicine and Surgery:
“The treatment of primary axillary hyperhidrosis can be rewarding using noninvasive microwave technology. Because the microwaves preferentially target the region of skin where the sweat glands reside, leading to localized thermolysis of the sweat glands, patients can now benefit from permanent targeted sweat reduction. The microwave treatment has been shown to be safe and effective in >6000 procedures to date.”
miraDry has been developed by Miramar Labs of Sunnyvale, California.
While miraDry is promising news for those who suffer with underarm sweating, it cannot be used to treat excessive sweating in other areas such as hands or feet because the procedure has been optimized to take into account the physical attributes of the underarms.
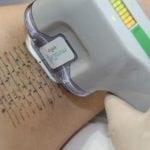
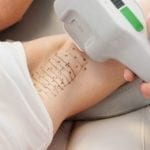
miraDry uses a non-invasive handheld device to deliver precisely controlled electromagnetic energy beneath the underarm skin to the specific area where sweat glands are located, resulting in thermolysis (decomposition by heat) of the sweat glands. While the sweat glands are being eliminated through electromagnetic technology, the top layers of the skin are simultaneously cooled and protected. Sweat glands are not believed to grow back after treatment so the effect can be seen almost immediately and results are lasting. For best results, two procedures spaced three months apart are recommended.
While Miramar Labs is the first company to gain FDA clearance to use electromagnetic energy to combat excessive sweating through eliminating or damaging sweat glands, the technology has been used safely in other areas of medicine for years. Established medical uses of electromagnetic energy include applications for cardiology, cosmetics, general surgery, urology, and oncology.
The miraDry procedure is performed in the physician’s office and typically takes one hour. Local anesthesia (usually lidocaine injections) will be administered before starting the treatment. Patients usually experience little to no discomfort during the procedure and there is minimal to no downtime afterwards. A mild over-the-counter pain medication and ice packs are generally recommended for a few days. Most patients are able to return to normal activities or work right after the procedure, and can typically resume exercise within several days.
Common and minor side effects of miraDry include underarm swelling, redness, and tenderness lasting for several days. Numbness and tingling can occur in the upper arm or armpit and may last for about 5 weeks. While sweating is an essential body function for temperature-control, the underarms house less than 2% of the body’s sweat glands. According to experts, the elimination of these sweat glands should, therefore, have no effect on body thermoregulation and compensatory sweating (sweating on other body parts, common after ETS surgery) has not been shown to be a concern. Depending on where you live, the cost of miraDry is approximately $3,000.
Next Steps
Underarm surgery is just one of numerous treatment options for excessive sweating (hyperhidrosis). Typically, experts recommend that patients try antiperspirantsfirst (over-the-counter “clinical” strength and/or prescription formulations) and then, if needed, progress to more invasive treatments.
Options include:
Iontophoresis (for hands and feet)
OnabotulinumtoxinA Injections (Botox®)
miraDry®(microwave technology)
Lasers
Oral medications
ETS (a last resort for palmar/hand sweating)
Use our Physician Finder to locate a healthcare provider near you who treats excessive sweating. We also have tips and tools to help you prepare for your appointment and navigate insurance and reimbursement. For the latest news about treatments, clinical trials, and special events sign-up for our newsletter. And please help to support our work by:
Making a donation
Purchasing the useful sweat-management products in our Deals and Discounts area
Liking us on Facebook and following us on Twitter
Spreading the word that excessive sweating is serious but treatable!
Research and References
Want to learn more about miraDry for the treatment of excessive sweating? Below are links to relevant articles and abstracts published in medical journals. More studies regarding other treatments can be found here:
The efficacy of a microwave device for treating axillary hyperhidrosis and osmidrosis in Asians: a preliminary study
Treatment of hyperhidrosis with microwave technology
A retrospective analysis of the treatment of axillary hyperhidrosis with a novel microwave technology
A randomized, blinded clinical evaluation of a novel microwave device for treating axillary hyperhidrosis: the dermatologic reduction in underarm perspiration study
Microwave thermolysis of sweat glands
Clinical evaluation of a microwave treatment for axillary hyperhidrosis: long-term follow-up
Clinical evaluation of a microwave device for treating axillary hyperhidrosis
A multi-center evaluation of the miradry system to treat subjects with axillary hyperhidrosis